PIPAC with conventional chemotherapy
A large systematic review covering 1,980 patients and 4,454 PIPAC procedures shows that PIPAC with standard chemotherapies is safe, feasible, and well tolerated, with low rates of severe complications. Objective histological tumor response was observed in ~62% of patients, while quality of life remained stable during treatment.
PIPAC with nanoparticles
In a Phase I study using nanoparticle albumin-bound paclitaxel (NAB-PTX), PIPAC demonstrated a favorable pharmacokinetic profile, progressive intratumoral drug accumulation, and encouraging anticancer activity. Systemic toxicity was limited, quality of life remained stable, and one-year survival reached 57%, supporting further clinical development.
PIPAC with siDNA
This proof-of-concept study showed that aerosolized Dbait (siDNA) penetrates tumor tissue up to 1 mm and induces strong intranuclear biological activity, outperforming conventional lavage. Tumor nodules demonstrated selective uptake and activation of DNA damage signaling, supporting clinical translation of nucleic-acid-based aerosols.
PIPAC with siRNA / mRNA
Preclinical studies demonstrated that mRNA and siRNA complexes remain stable under high pressure nebulization and can be effectively delivered to the peritoneal cavity using PIPAC. Local aerosol delivery resulted in confined intraperitoneal expression, highlighting PIPAC’s potential for RNA-based therapies.
PIPAC with immunotherapy
Nanoparticle-delivered DACHPt via PIPAC showed superior antitumor efficacy compared with free oxaliplatin in colorectal peritoneal carcinomatosis models. Beyond tumor control, treatment induced favorable immune modulation, including increased CD8⁺ T-cell infiltration and reduced immunosuppressive granulocytes.
PIPAC with oncolytic viruses (PIPAV)
Pressurized aerosol delivery of oncolytic adenoviruses proved technically feasible and well tolerated, with preserved viral viability after aerosolization. These results establish PIPAC as a promising platform for future locoregional virotherapy, though further optimization is needed to enhance in vivo efficacy.
Intraperitoneal delivery as pressurized aerosols can synergistically
be optimized by two factors : drug and device.
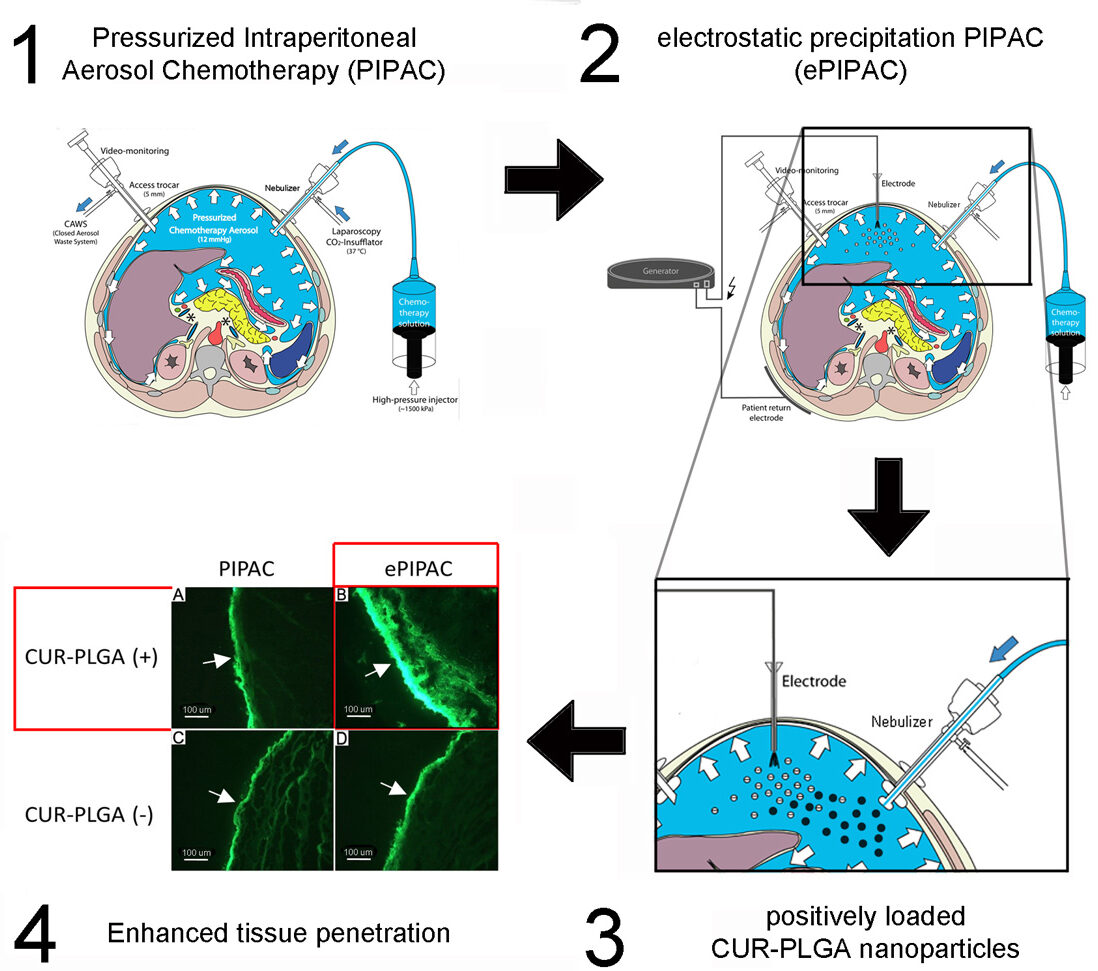
The case study presented here clearly demonstrates that the surface engineered nanoparticles designed for the peritoneal cavity microenvironment can create a superior impact when combined with a medical device technology such as PIPAC or ePIPAC. We compared cationic and anionic nanoparticles with crossed over medical technology group to show enhanced tissue uptake in an established ex vivo model of the human peritoneum. Medical science can work on both to get better pharmacological effects.